MSDS stands for Material Safety Data Sheet. A Material Safety Data Sheet (MSDS) is a document that contains information on the potential hazards and how to work safely with the chemical products, as well as safety precautions for storing, handling, and transporting chemicals and emergency procedures all related to the hazards of the material.
For proper identification of material hazards, a material safety data sheet (MSDS) should be prepared and supplied with each chemical so that its safety precautions can be well understood.
MSDSs are prepared by the supplier or manufacturer of the material. MSDS sheets are created for a variety of hazardous materials including compressed gases, flammable and combustible liquids, oxidizing materials, poisonous or infectious material, corrosive material, and dangerously reactive materials.
The Material Safety Data Sheet must be in the English language. MSDS revisions are required every 3 years or as soon as possible if new product information is available.
Chemical manufacturers and importers are required to develop a Material Safety Data Sheet for each hazardous chemical they produce or import. Employees must have an MSDS data sheet for each hazardous material that they use. MSDS are always used before using hazardous materials.
The MSDS must be readily available to the workers involved in handling, storing, and transporting hazardous chemicals. It provides guidance for each specific chemical’s such as:(1)Personal Protective Equipment (PPE) (2)First aid procedures(3)How to handle and storage.
An MSDS sheet is a safety document that gives the details of the toxicity, use, storage, handling, and emergency procedures of hazardous substances. The MSDS describes chemical safety and hazards that may be involved with the product and safety measures that should be taken in order to minimize or avoid adverse outcomes that may result from chemical exposure, chemicals in the workplace, improper storage or handling of a hazardous substance, and chemical hazards.
Material Safety Data Sheet information is intended to provide employees and emergency personnel with safety measures for handling or working with hazardous substances in a safe manner.
Table of Contents
Purpose of MSDS
The purpose of an MSDS is to ensure that all workers who handle chemicals have the hazard information they need to safely use, handle and store them. But nowadays SDS is used worldwide.
MSDS Contents
Every Material Safety Data Sheet has the following 9 categories or sections of information:
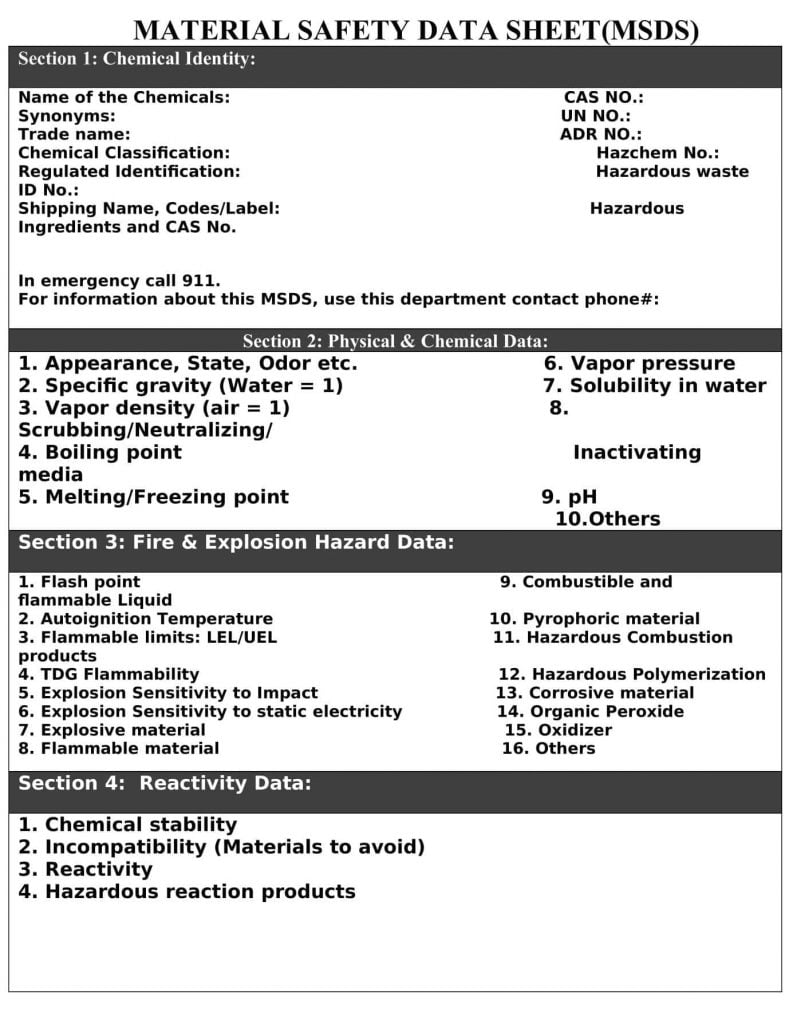
Section 1- Chemical Identity:
- Name of the Chemical
- Formula
- Synonyms
- Trade name
- Chemical Classification
- Regulated identification
- Shipping Name, Codes/Label
- CAS No.
- UN No.
- ADR No.
- Hazchem (EAC) No.
- Hazardous Waste ID No.
- Hazardous Ingredients and CAS No.
Section-2Physical & Chemical Data:
- Appearance, State, Odour etc.
- Specific gravity (Water = 1)
- Vapour density (air = 1)
- Boiling point
- Melting/Freezing point
- Vapour pressure
- Solubility in water
- Scrubbing/Neutralising/Inactivating media
- pH
- Others
Section-3 Fire & Explosion Hazard Data:
- Flashpoint
- Autoignition Temperature
- Flammable limits: LEL/UEL
- TDG Flammability
- Explosion Sensitivity to Impact
- Explosion Sensitivity to static electricity
- Explosive material
- Flammable material
- Combustible and flammable Liquid
- Pyrophoric material
- Hazardous Combustion Products
- Hazardous Polymerisation
- Corrosive material
- Organic Peroxide
- Oxidizer
- Others
Section-4 Reactivity Data:
- Chemical stability
- Incompatibility (Materials to avoid)
- Reactivity
- Hazardous reaction products
Section-5 Health Hazard Data:
- TLV (ACGIH)
- STEL/SET
- LC50 or LD50
- Odour threshold
- Carcinogen? Poison? Liberates poisonous fume?
- Routes of entry
- Body parts that may be affected
- Effects of exposure and symptoms
- Emergency and first aid treatment
- Engineering controls necessary for safe handling.
- NFPA Hazard signals 12. Special Health hazards.
Section-6 Precautions for Safe Handling and Use
- Ventilation required and type
- Personal protective equipment required and type
- Handling and storage precautions
Section-7 Emergency and First-aid Measure:
- Steps to be taken in case material is released or spilled.
- Waste disposal method for solid, liquid, and gaseous waste.
- Fire, extinguishing media, special procedures, and Unusual hazards.
- Exposure – First-aid measures. Antidotes, Dosages.
Section-8 Additional Information / References
Section-9 Manufacturer / Supplier’s Data:
- Name of Firm
- Mailing address
- Telephone/Telex/Fax Nos.
- Telegraphic address
- Contact person in an emergency
- Local bodies involved
- Standard packing
- Tremcard Details
- Other
Uses of Material Safety Data Sheet
For the better understanding and use of the Material Safety Data Sheet(MSDS), some terms are explained below:
1.Chemical Formula:
It is a symbolic representation of a chemical entity or relationship between elements, molecules and atoms. C2H2 benzene contains six atoms of carbons and six atoms of hydrogen in one molecule, group or ion. Thus, by formula, we can know the hazardous ingredient of a chemical.
2.Synonym:
Synonym Indicates the alternate name of the material. e.g. Dimethyl ketone or 2-Propanone for Acetone.
3.Trade Name:
Commercial name of the product.
4.Chemical Classification:
The general classification is organic or inorganic. Hazard wise classification can be flammable, explosive, toxic or poisonous, corrosive, reactive, infectious, oxidising, radioactive etc.
5.CAS No. :
It is the Chemical Abstracts Service number to provide a single unique identifier with naming the chemical, e.g. CAS No. for acetic acid is 64-19-7. It does not indicate the hazards of a material.
6. UN No. :
It is the United Nations four-digit number assigned to potentially hazardous material (e.g. Ammonia UN No. 1005) or Class of material (e.g. corrosive liquids UN No. 1760). These numbers are internationally recognised and used by emergency response personnel to identify material during transport emergencies. UN, Hazchem, NA and PIN numbers have the same uses.
7. Hazchem (EAC) No. :
Hazchem (hazardous chemical) Code or EAC (Emergency Action Code) is an emergency code confirmed by the Health & Safety Executive, UK. It consists of a number (I to 4) followed by one or two letters and signifies the type of fire extinguisher required, type of personal protective equipment required, whether the spillage should be contained or diluted with water, whether the material is reactive and whether evacuation of the surrounding area necessary. Hazchem No. of Sodium cyanide is 4X and that of Vinyl chloride is 2WE.
8. ADR No. :
It is an Agreement concerning the carriage of Dangerous goods by Road. This European agreement was arrived at Geneva by 19 European countries for the safety of international transport by road.
It deals with the classification of hazardous substances, their packaging, loading and unloading, transportation, and equipment. It gives hazard identification numbers like UN hazard class numbers.
9. Appearance, State, Odour:
Appearance includes colour. State means physical state – solid, liquid or gas. Odour indicates smell. The odour threshold is the minimum level (ppm) where the odour will start. If the odour threshold is lower than the permissible safe limit (e.g. TLV, STEL, IDLH or LC), the odour indicates the presence of gas and some safety margin is available to run away or to take a precautionary step.
But if it is higher, the gas becomes toxic or hazardous before its odour starts and this condition is risky. In that event a reliable gas detector is useful.
10. Specific Gravity (water = 1) :
It is the ratio of the density of a material to the density of water (which is I g/cc). Lighter material (Sp. gr. <l, e.g. benzene 0.88) will float, and heavier material (Sp. gr. >l, e.g. sulphuric acid 1.84) will sink. This information is useful for spill or fire control.
11. Vapour Density (air = 1) :
It is the vapour weight per unit volume. In MSDS it is given as the ratio of the density of a gas or vapour to the density of air. The air density is 1.293 gm/l, but here it is considered as 1 for easy comparison of gases.
12. Boiling Point:
It is that temperature at which the material changes from a liquid to a gas. Below this point, the liquid can evaporate to form vapour but at the BP the change from liquid to vapour is faster. This increases the vapour concentration and its pressure. This condition poses a higher risk of fire, explosion or toxicity.
13. Thermal Decomposition Products:
If the material decomposes (breaks down) without boiling, the temperature at which it decomposes is given with the word “dec’. Some of the decomposition products are hazardous. The thermal decomposition products may be quite different from the chemicals formed by burning the same material (hazardous combustion products).
Information regarding thermal decomposition is useful to design a ventilation system where a material may be heated.
14. Hazardous Decomposition Products:
They are formed when a material decomposes (without heating) because it is unstable or reacts with common material like water or air (oxygen). This information is useful to design storage and handling procedures.
For example, phosgene decomposes into corrosive and toxic fumes of HCI and CO because of heating or coming into contact with water or steam. Here HCI and CO are hazardous decomposition products.
15. Hazardous Combustion Products:
These are the chemicals that are formed when a material burns. They may be toxic, flammable, smoke, carbon particles, or other hazards. Their amount varies according to temperature and oxygen (air) available.
They may be different from the thermal decomposition products. This information is useful to decide the firefighting material and procedure.
16. Melting Point :
It is that temperature at which a solid material melts and becomes a liquid. This information is useful for storage and handling purpose.
17. Freezing Point :
It is that temperature at which a liquid material freezes and becomes solid. This information is useful for storage and handling purpose. A frozen material may burst into a container.
18. Vapour Pressure :
It is the pressure (mm of Hg) upon the atmosphere of the vapour of a material at a fixed temperature (e.g. 20 °C). Higher vapour pressure indicates higher concentration and therefore higher hazard due to fire or inhalation.
19. Solubility :
It is the ability of a material to dissolve in water or another liquid (solvent). It may be expressed as a ratio or described by words like insoluble, very soluble, sparingly soluble, or miscible. This information is useful to decide a scrubbing media, spill control or firefighting material and procedure. Such solvent should not be hazardous.
20. Scrubbing neutralising or inactivating media:
These are those materials (liquids) that dissolve or react with the hazardous material (gas, liquid or solid) to diminish its hazardous exposure e.g. caustic, lime, water, etc. If this is not possible, the proper absorbent may be used e.g. sand, sponge rubber, etc.
21. pH :
It is a measure of the acidity or alkalinity (basicity) of material when dissolved in water. It is expressed on a scale from 0 to 14 as under:
| pH 0-2 | Strong acidic |
| pH 3-5 | Weak acidic |
| pH 6-8 | Neutral |
| pH 9-11 | Weak basic |
| pH 12-14 | Strong basic |
22. Flash Point :
It is the lowest temperature at which a material gives off enough vapour near its surface to form a flammable air vapour (gas) mixture so that it can be ignited if a spark is available.
The lower flesh point indicates a higher hazard as it can cause fire at a lower temperature. It is expressed as Closed Cup (CC) or Open Cup (OC). CC value is slightly less than the OC value.
23. Auto-ignition Temperature:
It is the lowest temperature at which a material begins to burn in the air without any contact of spark or flame. During heating, if the material decomposes, the decomposed chemical may auto-ignite at some other temperature. Different test methods give different auto-ignition temperatures for the same material.
Therefore, this value is an estimate. The material should be stored, processed, or handled well below its auto-ignition temperature to avoid the risk of self-fire or explosion. Substances liable to spontaneous combustion are those liable to spontaneous heating under normal conditions or to heating upon contact with air and are then liable to catch fire.
24. Flammable or Explosive Limits (LEI/UEL) :
The lowest concentration (percentage in the air) of gas or vapour which will burn or explode if ignited, is called the Lower Explosive (or Flammable) Limit i.e. LEL or LFL. The upper concentration (percentage in the air) of gas or vapour which will burn or explode if ignited, is called the Upper Explosive (or Flammable) Limit i.e. UEL or UFL.
The range between LEL and UEL is called the Explosive (or Flammable) Range. The fire or explosion risk lies within this range but not out of it. Below LEL the gas-air mixture is too lean to ignite and above UEL it is too rich to ignite.
However, the concentration above UEL should be considered dangerous as due to the entrainment of fresh air, it may be diluted and enter the explosive range. Similarly, after LEL if the gas discharge is continued in the same air, it can also enter the explosive range.
Thus, the explosive range can be reached depending on the flow of gas and air affecting their concentration. This information is useful to avoid the conditions leading to the explosive range and to ascertain it before allowing any person to enter any vessel or confined space where such air-gas mixture is suspected.
25. TDG Flammability :
Transport of Dangerous Goods (TDG) classifies the materials according to their flammability as under-
- 2.1 Flammable gas.
- 3 Flammable liquid (Subclasses 3.1, 3.2, and 3.3 based on flash point).
- 4.1 Flammable solid.
- 4.2 Spontaneously combustible material.
- 4.3 Material that gives off a flammable gas in contact with water.
26. Explosion Data (Sensitivity) :
It gives explosive properties of a material e.g. low, moderate or high. It gives two types of sensitivity:
Explosion Sensitivity to Impact – It indicates whether or not the material will burn or explode on shock or friction, and
Explosion Sensitivity to Static Electricity – It indicates how readily the material can be ignited by an electric spark or static discharge.
27. Explosive Material:
Explosive material is that material that can explode on impact or by electric spark.
28. Combustible and Flammable Material:
Flammable solid, liquid, or gas that can catch fire and burn rapidly or explosively are flammable materials. The terms combustible and flammable both indicate the ability of a material to burn.
29. Corrosive Material:
It can attack metals or human tissues such as skin or eyes. Structure or metal container may become weak and eventually collapse or leak. Skin, eyes, or other body parts can be badly affected (burning) by corrosive materials. Acids, halogen gases, chlorides, caustic, phenol, etc. are corrosive.
30. Hazardous Polymerisation:
A polymer is a natural or man-made material formed by combining units called monomers, into long chains, e.g. styrene is the monomer for polystyrene. Polymerization is the process of forming a polymer by combining monomers into long chains. Uncontrolled polymerization can be hazardous, as it can cause heat, pressure, or explosion.
Some chemicals can polymerize on their own without warming, others upon contact with water, air, or common chemicals. Vinyl chloride rapidly polymerizes in presence of light, air, or heat. Therefore polymerizing conditions should be controlled properly; inhibitors are normally added to products to reduce or eliminate the possibility of hazardous polymerization.
31. Pyrophoric Material :
Any liquid or solid that will ignite spontaneously in air at about 54.4 °C (130 °F). Titanium dichloride and phosphorous are examples of pyrophoric solids, tributyl aluminium, and related compounds are pyrophoric liquids. Sodium, butyllithium, and lithium hydride are spontaneously flammable in moist air as they react exothermically with water.
Such materials must be stored in inert gas or under kerosene. Some alloys (barium, misc. metal) are called pyrophoric because they spark when slight friction is applied. Pyrotechnic materials mean fireworks.
Catalysts of pyrophoric material which can burn in normal air, are replaced in the atmosphere of nitrogen blanketing. The workers have to wear self-breathing apparatus while doing such a job because, in an atmosphere of about 90% nitrogen, oxygen is insufficient for breathing.
32. Oxidiser and Peroxide:
It is a compound that spontaneously evolves oxygen either at room temperature or under slight heating. Oxidizers include peroxides, chlorates, perchlorates, nitrates, and permanganates. These can react vigorously at ambient temperatures when stored near or in contact with reducing materials (that will remove oxygen or add hydrogen) such as cellulosic and other organic compounds. Storage areas should be well ventilated and kept as cool as possible.
Peroxides release atomic oxygen readily. They pose fire hazards in contact with combustible materials, especially under high-temperature conditions. They are used as oxidizing agents, bleaching agents, and initiators of polymerization. Oxidizing substances are not necessarily combustible in themselves but by giving oxygen they contribute to the combustion of other materials.
33. Chemical Stability :
A stable compound does not easily decompose or react readily. Chemical stability is the ability of a material to remain unchanged in the presence of heat, moisture, or air. An unstable compound may decompose, polymerize, burner explode under normal environmental conditions.
Special precautions are required to store or handle unstable materials. For example, CS2 decomposes in light and burns due to heat, spark, flame, or friction and gives off toxic fumes of SOx. Caprolactam liberates NOx fumes due to heating. TNT explodes due to heavy shock or by heating. Thus conditions disturbing stability must be known.
34. Incompatibility:
Compatibility means the ability of two or more materials to exist in close and permanent association indefinitely. Liquids and solids are compatible if the solid is soluble in the liquid. Water is compatible with alcohol (because it is miscible) but not with gasoline (e.g. petrol).
35. Reactivity:
Two or more chemicals can react with each other and give reaction products, e.g. 2H2 + 02 = 2H20. A single chemical can react with air or water (which are also chemicals) and give the product, e.g. phosphorous burns in air and gives its oxides (P2O3, P2O5), sulfur burns and gives SO2 etc.
In MSDS we are concerned with the hazardous reaction or reactive material which can cause fire, explosion, toxic release, or violent reaction with air, water, or common chemicals or under environmental conditions. Phosphorous, CS, Sodium metal, acids, etc. are known for their reactivity. This information is useful for storage, handling, and process safety purposes.
36. Hazardous Reaction Products:
These must be known for the safety of processes, workers, and the environment. Here products are more important than the reaction because of their hazardous nature, e.g. Chlorine reacts with alcohol and forms explosive alkyl hypochlorite.
If toxic fumes are to be generated, scrubbers are required, if flammable vapours are generated, inert gas blanketing is required, and earthing of the vessel also becomes necessary. If reaction products are highly poisonous like NaCN, HCN, etc., they are to be handled in a closed system.
37. Tremcard:
Transport Emergency Cards are to be given to the drivers carrying dangerous goods for emergency information which may be needed at any time during the journey. The cards contain short information on the nature of chemicals, hazards involved, protective devices, emergency action for fire, spillage, leakage, first-aid, etc.
Material Safety Data Sheet/MSDS Sheet

What is SDS?
SDS stands for (SAFETY DATA SHEET) formerly known as Material safety data sheet (MSDS). Some countries may still call this document Material Safety Data Sheet (MSDS). The older MSDS formats could vary from source to source within a country depending on its requirements, but the newer Safety data sheet (SDS)format is internationally standardized.SDS has 16 sections:
SDS SECTIONS
SECTION 1: Identification of the substance/mixture and of the company/undertaking
- Product identifier
- Relevant identified uses of the substance or mixture and uses advised against
- Details of the supplier of the safety data sheet
- Emergency telephone number
SECTION 2: Hazards identification
- Classification of the substance or mixture
- Label elements
- Other hazards
SECTION 3: Composition/information on ingredients
- Substances
- Mixtures
SECTION 4: First aid measures
- Description of first aid measures.
- Most important symptoms and effects, both acute and delayed.
- Indication of any immediate medical attention and special treatment needed.
SECTION 5: Firefighting measures
- Extinguishing media
- Special hazards arising from the substance or mixture
- Advice for firefighters
SECTION 6: Accidental release measure
- Personal precautions, protective equipment, and emergency procedures
- Environmental precautions
- Methods and material for containment and cleaning up
- Reference to other sections
SECTION 7: Handling and storage
- Precautions for safe handling
- Conditions for safe storage, including any incompatibilities
- Specific end use(s)
SECTION 8: Exposure controls/personal protection
- Control parameters
- Exposure controls
SECTION 9: Physical and chemical properties
- Information on basic physical and chemical properties
- Other information
SECTION 10: Stability and reactivity
- Reactivity
- Chemical stability
- Possibility of hazardous reactions
- Conditions to avoid
- Incompatible materials
- Hazardous decomposition products
SECTION 11: Toxicological information
- Information on toxicological effects.
SECTION 12: Ecological information
- Toxicity
- Persistence and degradability
- Bio accumulative potential
- Mobility in soil
- Results of PBT and vPvB assessment
- Other adverse effects
SECTION 13: Disposal considerations
- Waste treatment methods.
SECTION 14: Transport information
- UN number
- UN proper shipping name
- Transport hazard class(es)
- Packing group
- Environmental hazards
- Special precautions for user.
SECTION 15: Regulatory information
- Safety, health, and environmental regulations/legislation specific for the substance or mixture
- Chemical safety assessment
SECTION 16: Other information
- Date of the latest revision of the SDS.
Safety data sheet example:






